BIOLOGY'S NEXT TOP MODEL: ORGANOIDS
YOUNG, CONFIDENT, AND TURNING HEADS ON THE RUNWAY.
Human beings make for great scientists, but unsatisfactory biological test subjects. Think about it for a minute - if you were a researcher, how would you study an organism that is genetically diverse and takes decades to mature? These are only a couple of the many challenges that biologists face when studying human health and disease.
As a result, scientists often use simplified models to study human biology. These strategies fall into one of two broad categories: in vitro or in vivo studies**. In vitro* studies utilize cell lines, which are preparations of cells that grow easily in a laboratory setting. However, many cell lines are derived from tumor cells riddled with genetic mutations, and thus do not always behave like healthy cells.
In vivo experiments use lab animals like mice, which can be used to study development, behavior, response to infection, and lots of other questions that cannot be answered by studying cells in a dish. But, as you might imagine, there are significant biological differences between humans and lab animals. After all, there is a reason scientists have had far more success curing cancer in mice than in humans.
In recent years, biologists have developed a new cell culture system that combines the speed and ease of in vitro work with biological functions normally only found in vivo. Organoids are three-dimensional cell cultures that look and behave like the tissue or organ that they were derived from.
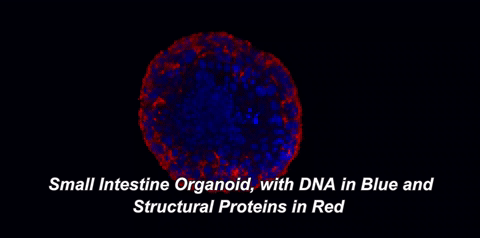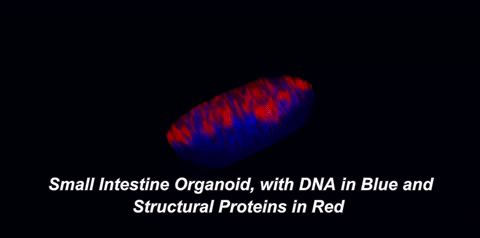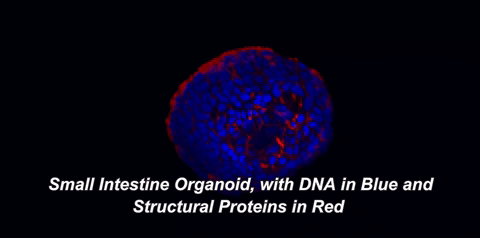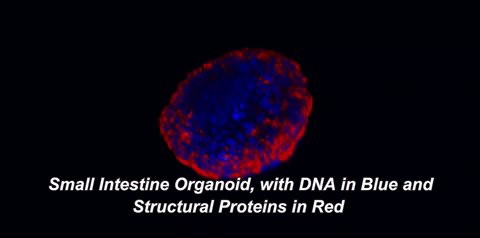
Here is what that means in plain English: throughout your body, there are special cells called stem cells which can divide equally into two new stem cells (a process called self-renewal) or can divide into one stem cell and a cell with more specialized functions, like an immune cell or a muscle cell (a process called differentiation). In 2009, a team of Dutch scientists led by Hans Clevers at the University Medical Center of Utrecht showed that small intestinal stem cells could be grown into 3D structures that contained all cell types normally found in the intestine1. These gut organoids sort of look like balls with protruding buds. On the inside of this ball, called the lumen, are dead cells; our guts work the same way, as the cells that line them only live three to five days before undergoing a programmed death called apoptosis. So, how are scientists able to grow organoids for years? The answer lies in those small buds, or crypts, which are rich in intestinal stem cells.
One of two key criteria for growing organoids is the use of a nutrient-rich solution that keeps these precious stem cells alive. In the body, stem cells exist within a special environment called the stem cell niche that contains critical signals for their survival. Hans Clevers and collaborators were among the first to identify the signals within the stem cell niche, as well as how to provide these signals when growing stem cells in the lab. The other key is the addition of an extracellular matrix (ECM). This matrix, just like the one in the hit movie starring Keanu Reeves, is an omnipresent force that controls our lives, but with fewer epic, slow-motion battle scenes. The ECM is made of proteins and sugars that provide structure, support, and signals to our cells. When organoids are grown in ECM, they develop a cellular lining, or epithelium, which is polarized. The term "polarized" means that the surface of the cells facing the inside of the organoid is covered with different molecules than the surface facing the outside. Epithelial cells in the body are also polarized. For example, the side of your intestinal epithelium that faces incoming food absorbs nutrients and deal with gut bacteria, while the other side interacts with nearby immune cells. It makes sense that each side would possess distinct molecules to carry out its distinct functions.
Organoids have now been grown from the liver, pancreas, stomach, prostate and even the brain! Try wrapping your mind around that, pun intended. Okay, so organoids are interesting, but why are they important? One key reason is that organoids can be grown from the tissue of specific individuals. This is pivotal, because it means that a researcher can generate a "biobank" of organoids from a diverse patient population.
Here is a great example. A group of Dutch scientists generated small intestinal organoids from patients with cystic fibrosis (CF), a disease in which a defective chloride ion channel causes a life-threatening buildup of mucus in the respiratory and digestive tracts. Interestingly, the researchers observed that the response of organoids to drugs that improved channel function depended on the specific channel mutation that the patient had2. Maybe, they thought, organoids could be used to predict what drug would work best for each patient. Early data suggest that this is true; a few CF patients have already been successfully treated with this approach, and larger-scale studies are now in progress.
Organoid biobanks could lead to personalized medicines for all sorts of diseases, and the Clevers lab is now building a biobank for colon cancer4. Doctors routinely take biopsies during colon cancer diagnosis, and researchers can use biopsies to produce organoids from the tumor as well as from surrounding healthy tissue. By comparing normal and tumor organoids, the Clevers group identified mutations unique to the tumor. Those tumor organoids were then screened against a panel of drugs to see which drugs effectively inhibited growth. As with CF, trials are now underway to test how well these drugs work in patients.
In the coming years, the horizons for organoid research will continue expanding. One of the next steps for this field will be growing organoids which consist of epithelial cells, alongside other cell types present in their local environment. For example, intestinal epithelial cells interact with immune cells as well as a vast population of gut resident bacteria known as the microbiome. Incorporating immune cells and the microbiome into intestinal organoids will allow researchers to precisely understand how epithelial cells, bacteria, and the immune system work together to regulate the gut. Another area of unrealized potential is the use of organoids for transplantation. While most organoids are currently no larger than a few millimeters, scientists may one day be able to grow organoids from a patient's own tissue, repair disease-causing genes, and re-implant those organoids back into the patient. Remember the CF story? Well, scientists have already shown that the gene responsible for the defective chloride channel can be fixed in patient organoids4.
In summary, biologists are always on the hunt for new and better ways to understand the complexities of the human body. Organoids have already emerged as a powerful system that can complement existing animal and cell line studies, and the field is still in its infancy. Stay tuned for more exciting updates!
In vivo is Latin for "within the living," and refers to the study of intact, living organisms. In contrast, in vitro, which means "within the glass," is the study of biological processes outside of an organism. The most recognizable example of in vitro science is in vitro fertilization (IVF), which doctors use to help couples struggling to have a child. Because sperm and egg are mixed together in a dish instead of, shall we say, the "natural" way, IVF is an in vitro process.
REFERENCES
Sato, Toshiro, et al. "Single Lgr5 stem cells build crypt villus structures in vitro without a mesenchymal niche." Nature 459.7244 (2009): 262-265.
Dekkers, Johanna F., et al. "A functional CFTR assay using primary cystic fibrosis intestinal organoids." Nature Medicine 19.7 (2013): 939-945.
Van de Wetering, Marc, et al. "Prospective derivation of a living organoid biobank of colorectal cancer patients." Cell 161.4 (2015): 933-945. APA
Schwank, Gerald, et al. "Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients." Cell Stem Cell 13.6 (2013): 653-658.